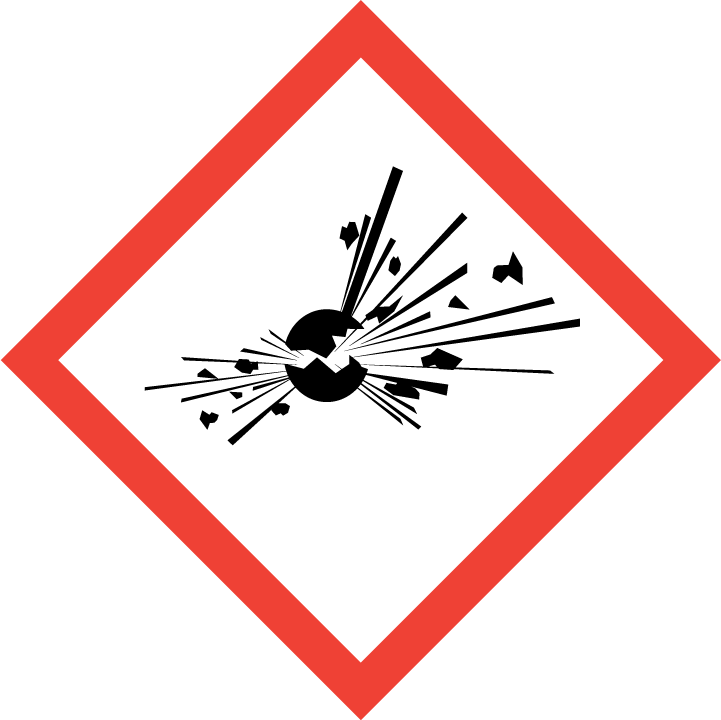
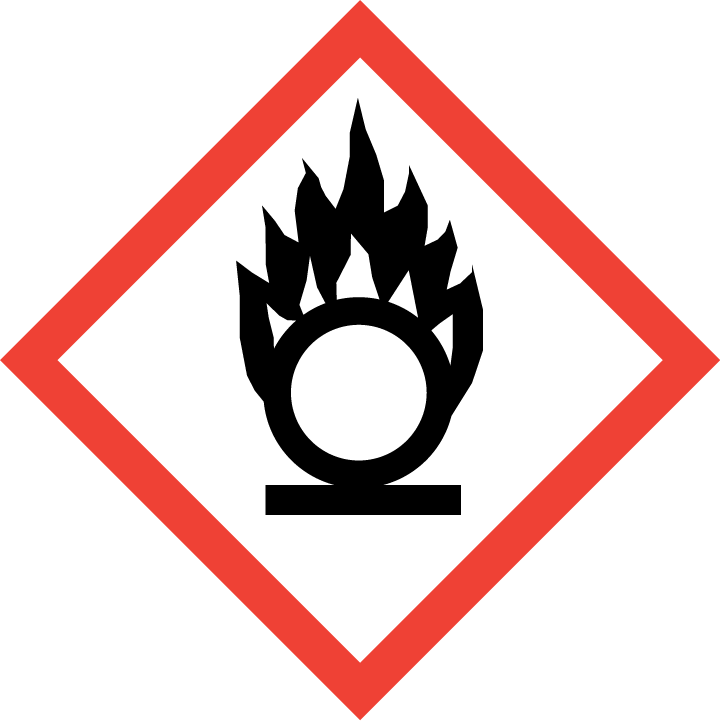
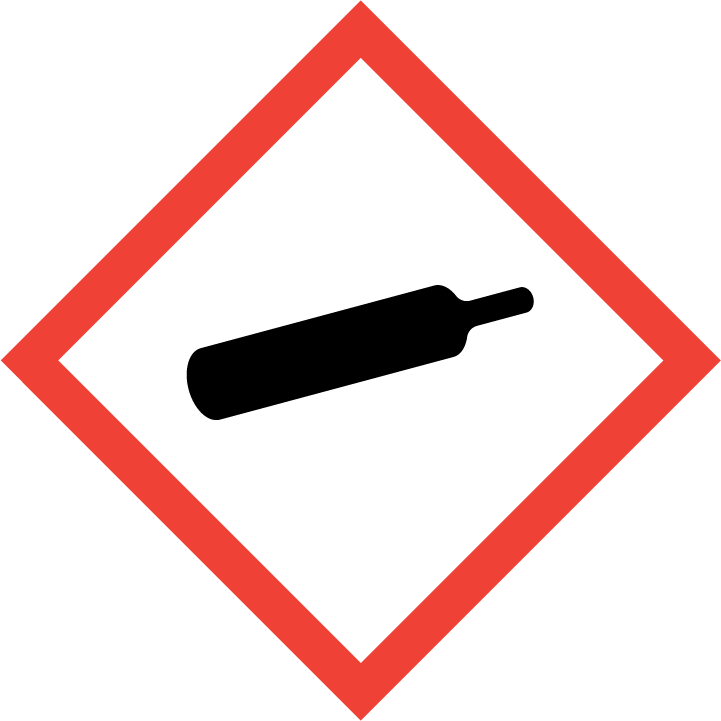
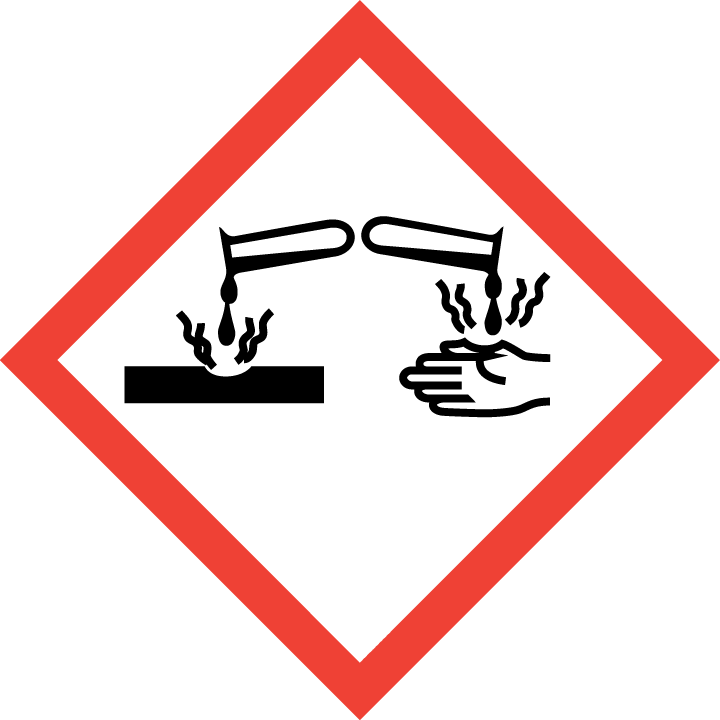
If a detergent is classified as dangerous, regulations relating to the market of dangerous chemicals must be considered primarily (acquiring marketing authorization for dangerous chemicals, making of a Safety Data Sheet, label, applying to the Chemicals Office or acquiring an authorization number, reporting annual volumes etc).
All detergents (dangerous and non-dangerous) must be correctly marked. If the concentration of ingredients from the list in Annex VII of the Regulation (EC) No. 648/2004 exceeds 0.2%, they have to be stated on the packages of detergents intended for general use.
The content of enzymes, optical brighteners, fragrances and disinfection ingredients should be stated regardless of their concentration.
Special rules also apply to stating allergenic fragrances classified in Annex III of the Regulation (EC) 1223/2009 on cosmetic products. They always have to be stated on the outer packaging with the actual names from the Regulation on cosmetic products when their concentration in the product exceeds 0.01%.
Surfactants must meet the requirements about biodegradability of surfactants within the meaning of the Regulation (EC) No. 648/2004 on detergents.
Conditions for the use of phosphates in other phosphoric compounds in household detergents for washing clothes and machine washing of dishes.