Today, let's look at the third condition (c), which, unlike the first two conditions, refers to the exact concentration of the ingredient.
This condition says that the exact concentration of the ingredient was submitted, but there was a change in concentration that exceeded the ranges listed in Table 3.
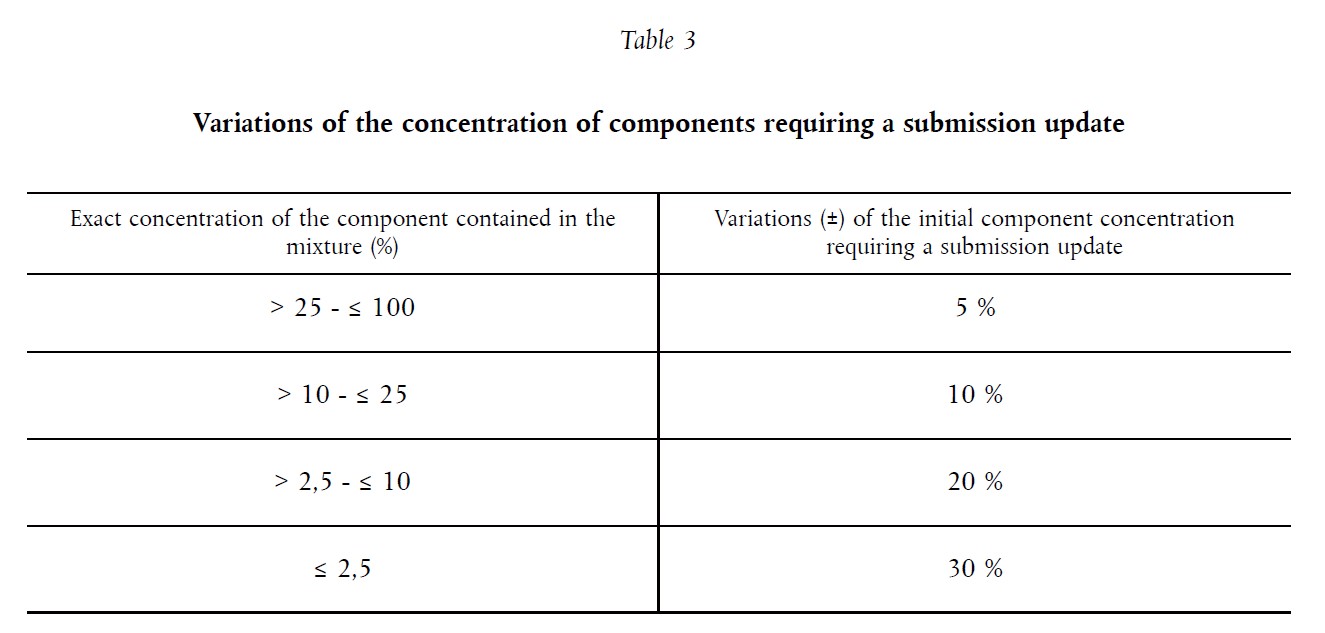
Table 3 below shows you the guidelines and limits to consider when assessing whether a new UFI is needed:
Let’s have a look at a few examples for better understanding:
- The concentration of the substance classified with H319 is 20%. This concentration was also submitted in the first submission (the exact concentration was used instead of a range). New concentration is 25%.
Answer: According to the Table 3, the maximum modification of the initial concentration is ±10 %. The new concentration represents a 25% change. Therefore, a NEW UFI must be generated.
- Concentration of substance is 72%. This concentration was submitted in the first submission. Answer: According to Table 3, a maximum discrepancy of ± 5 % is allowed. In this case, a new UFI must be generated if the new concentration of the substance is below 68.4 % or above 75.6 %.
The compound contains 4 substances (A, B, C, D). Their exact concentration was submitted (second column) as you can see in the table below.
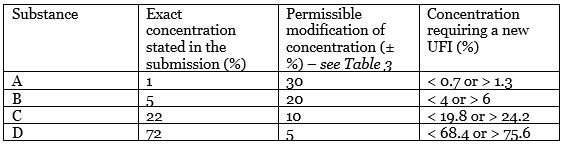
The third column then lists the permitted deviations with respect to the previously stated exact concentration. It means you need to create a new UFI if the concentration changes below or above the value stated in the last column.
I hope the examples are clear enough, and helped you understand the requirements related to UFI. In any case, you can contact me and we will have a chat about your particular challenge.
Here are the links to the first and second post on this topic.
If you don't know where to start, or you don't have the time to effectively and correctly implement (and update) this PCN notification, it might be time to write to me at simona.miklavcic@bens-consulting.eu.






 Back to posts
Back to posts

