Previously we had a look at the first of the three conditions based on which a new UFI must be created.
Let's have a look at condition (b) today. In this case, a new UFI must be created, if modification of concentration of an ingredient in the compound exceeds the concentration range stated in the initial submission.
In the evaluation it is necessary to check how the modification of concentration influences the limits defined in the tables.
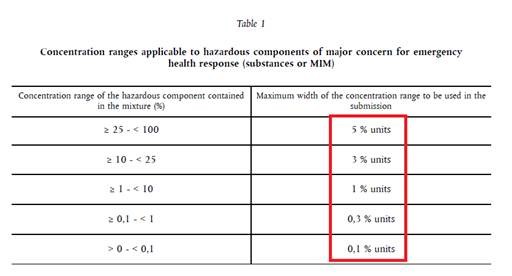
Firstly, we have Table 1. This applies for substances having the following H-statements: H318, H314, H314.1A, H314.1B, H314.1C, H300.1, H300.2 H301, H302, H310.1, H310.2, H311, H312, H330.1, H330.2, H331, H332, H370, H371, H372, H373
Let’s have a look at two examples to understand the topic better.
- Let’s say that your compound contains 20 % of substance with H319. In the initial submission, the chosen concentration range was 18 % to 21 %.
Answer: If the new concentration of substance is below 18 % or over 21 %, it is necessary to define a new UFI. So, a new UFI must be generated if the new concentration of substance falls outside the concentration range defined in the initial submission.
- Concentration of the substance classified with H314 is 20 %. In the initial submission, the chosen concentration range was 20 % to 21 %.
Answer: The new concentration of this substance is 19 %. As the new concentration of this substance in the compound is outside the range of the initial submission, a new UFI must be generated.
Therefore, we suggest choosing in the initial submission the maximum permitted concentration range. In this case, that is 3 units (see Table 1), e.g. from 17 % to 20 % or 18 % to 21 % or 19 % to 22 %, etc. If the new concentration falls within the chosen range, it is not necessary to generate a new UFI.
And now, Table 2. This applies for substances with other H-statements and substances that are not classified as hazardous.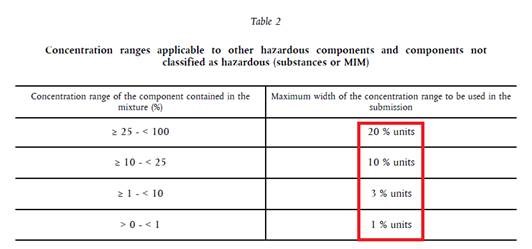
Let’s see in practice how to assess whether a new UFI is needed:
- Concentration of the substance classified with H319 is 20 %. In the initial submission, the chosen concentration range was 18 % to 24 %.
Answer: If the new concentration of substance is below 18 % or over 24 %, it is necessary to define a new UFI. So, a new UFI must be generated if the new concentration of the substance falls outside the concentration range defined in the initial submission.
- Concentration of the substance that is not classified as hazardous is 20 %. In the initial submission, the chosen concentration range was 20 % to 21 %. New concentration of this substance is 19 %.
Answer: As the new concentration of this substance in the compound is outside the range of the initial submission, a new UFI must be generated. Therefore, we suggest choosing in the initial submission the maximum permitted concentration range. In this case, that is 10 units (see Table 2), e.g. from 14 % to 24 %. If the new concentration of a hazardous substance falls within this range, it is not necessary to generate a new UFI.
- A modification of concentration of perfume, which only contains H317, from 1 % to 4.9 %.
Answer: In this case, a special rule applies instead of Table 2. For perfume or fragrance ingredients that are not classified or are only classified in terms of skin sensitization (H317) or represent a hazard of aspiration (H304), it is not necessary to submit information on concentration provided that the total concentration does not exceed 5 %. Therefore, it is not necessary to create a new UFI in this particular case.
We have come to an end of the second post.
If you have missed the first one, you can find it at this link. The third post in the series on the topic of when is it necessary to create a new UFI will follow soon.
If, after reading this, you have any questions, do not hesitate to send them to me. In many cases your questions provide the best input for writing on topics that you are interested in.






 Back to posts
Back to posts

