Restriction 68 of Annex XVII on perfluorocarboxylic acids containing 9 to 14 carbon atoms in the chain (C9-C14 PFCAs), their salts and C9-C14 PFCA-related substances are specified in COMMISSION REGULATION (EU) 2021/1297 – with the general deadline 25 February 2023.
“Linear and branched perfluorocarboxylic acids containing 9 to 14 carbon atoms in the chain (‘C9-C14 PFCAs’), their salts and C9-C14 PFCA-related substances (C9-C14 PFCA-related substances are substances that, based on their molecular structure are considered to have the potential to degrade or be transformed to C9-C14 PFCAs) currently mainly occur in the Union as unintended by-products during the manufacture of perfluorinated and polyfluorinated substances containing a carbon chain of less than nine carbon atoms, such as perfluorooctanoic acid (PFOA).
Furthermore, it is possible that companies may consider the use of C9-C14 PFCAs, their salts and C9-C14 PFCA- related substances as substitutes for PFOA, its salts and related substances in the future, especially after the Union Law restrictions on PFOA become applicable. Thus, it is necessary to prevent future possible manufacturing and use resulting in increasing releases into the environment.”
This introduces a restriction for these types of substances:
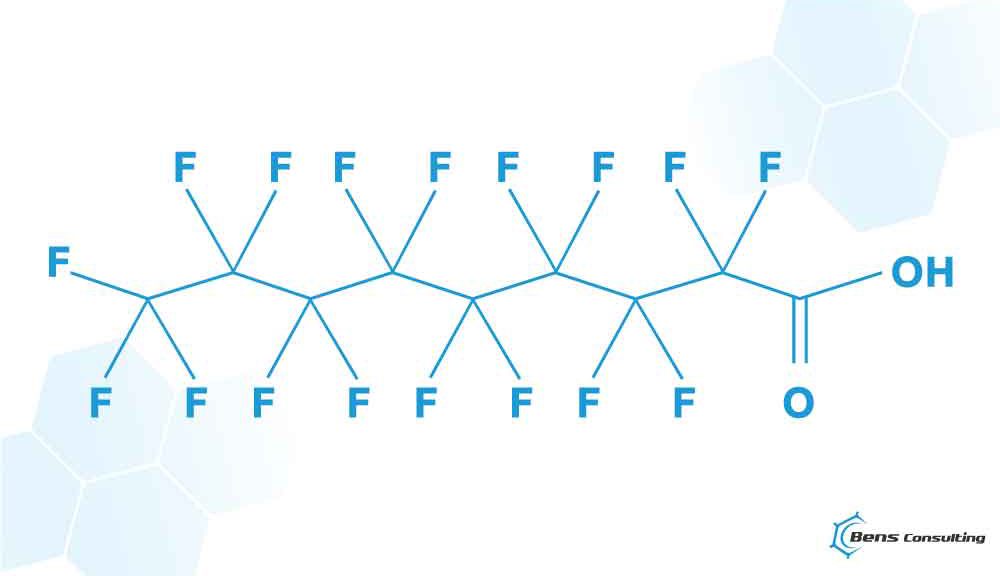
‘68. Linear and branched perfluorocarboxylic acids of the formula CnF2n +1-C(=O)OH where n = 8, 9, 10, 11, 12, or 13 (C9-C14 PFCAs), including their salts, and any combinations thereof;
Any C9-C14 PFCA-related substance having a perfluoro group with the formula CnF2n +1- directly attached to another carbon atom, where n = 8, 9, 10, 11, 12, or 13, including their salts and any combinations thereof;
Any C9-C14 PFCA-related substance having a perfluoro group with the formula CnF2n +1- that it is not directly attached to another carbon atom, where n = 9, 10, 11, 12, 13 or 14 as one of the structural elements, including their salts and any combinations thereof.
The following substances are excluded from this designation
—CnF2n +1-X, where X = F, Cl, or Br where n = 9, 10, 11, 12, 13 or 14, including any combinations thereof,
—CnF2n +1-C(= O)OX' where n> 13 and X'=any group, including salts.
You can find some of the CAS and EC numbers that fall under this restriction here on ECHA.
| Name | EC no. | CAS no. |
|---|---|---|
| Pentacosafluorotridecanoic acid | 276-745-2 | 72629-94-8 |
| Perfluorononan-1-oic acid | 206-801-3 | 375-95-1 |
| Henicosafluoroundecanoic acid | 218-165-4 | 2058-94-8 |
| Tricosafluorododecanoic acid | 206-203-2 | 307-55-1 |
| Nonadecafluorodecanoic acid | 206-400-3 | 335-76-2 |
| Heptacosafluorotetradecanoic acid | 206-803-4 | 376-06-7 |
So, here goes the restriction:
- Shall not be manufactured, or placed on the market as substances on their own from 25 February 2023.
- Shall not, from 25 February 2023, be used in, or placed on the market in:
(a) another substance, as a constituent;
(b) a mixture;
(c) an article, except if the concentration in the substance, the mixture, or the article is below 25 ppb for the sum of C9-C14 PFCAs and their salts or 260 ppb for the sum of C9-C14 PFCA-related substances.
As you can see, this is a quite a strict general restriction.
However, there are quite many derogations to the restriction with different deadlines to keep in mind. It is a long list, but worth reading if you use/place on the market mixtures or articles containing such substances. If you do not have the time to read all this, just jump over to the last part of my article where I give advice on how to deal with this restriction.
Here go the derogations:
3. By way of derogation to paragraph 2, the concentration limit shall be 10 ppm for the sum of C9-C14 PFCAs, their salts and C9-C14 PFCA related substances, where they are present in a substance to be used as a transported isolated intermediate, provided that the conditions in points (a) to (f) of Article 18(4) of this Regulation are met for the manufacturing of fluorochemicals with a perfluoro carbon chain length equal to or shorter than 6 atoms. The Commission shall review this limit no later than 25 August 2023.
- Paragraph 2 shall apply from 4 July 2023 to:
(i)textiles for oil- and water-repellency for the protection of workers from dangerous liquids that comprise risks to their health and safety;
(ii)the manufacture of polytetrafluoroethylene (PTFE) and polyvinylidene fluoride (PVDF) for the production of:
—high performance, corrosion resistant gas filter membranes, water filter membranes and membranes for medical textiles;
—industrial waste heat exchanger equipment;
—industrial sealants capable of preventing leakage of volatile organic compounds and PM 2,5 particulates
- By way of derogation to paragraph 2, the use of C9-C14 PFCAs, their salts and C9-C14 PFCA-related substances shall be allowed until 4 July 2025 for:
(i)photolithography or etch processes in semiconductor manufacturing;
(ii)photographic coatings applied to films;
(iii)invasive and implantable medical devices;
(iv)fire-fighting foam for liquid fuel vapour suppression and liquid fuel fire (Class B fires) already installed in systems, including both mobile and fixed systems, subject to the following conditions:
—fire-fighting foam that contains or may contain C9-C14 PFCAs, their salts and C9-C14 PFCA-related substances shall not be used for training;
—fire-fighting foam that contains or may contain C9-C14 PFCAs, their salts and C9-C14 PFCA-related substances shall not be used for testing unless all releases are contained;
—from 1 January 2023, uses of fire-fighting foam that contains or may contain C9-C14 PFCAs, their salts and C9-C14 PFCA-related substances shall only be allowed to sites where all releases can be contained;
—fire-fighting foam stockpiles that contain or may contain C9-C14 PFCAs, their salts and C9-C14 PFCA-related substances shall be managed in accordance with Article 5 of Regulation (EU) 2019/1021.
- Paragraph 2(c) shall not apply to articles placed on the market before 25 February 2023.
- Paragraph 2 shall not apply to the can coating for pressurised metered-dose inhalers until 25 August 2028.
- Paragraph 2 (c) shall apply from 31 December 2023 to:
(a) semiconductors on their own;
(b) semiconductors incorporated in semi-finished and finished electronic equipment. - Paragraph 2(c) shall apply from 31 December 2030 to semiconductors used in spare or replacement parts for finished electronic equipment placed on the market before 31 December 2023.
- Until 25 August 2024, the concentration limit referred to in paragraph 2 shall be 2 000 ppb for the sum of C9-C14 PFCAs in fluoroplastics and fluoroelastomers that contain perfluoroalkoxy groups. From 25 August 2024, the concentration limit shall be 100 ppb for the sum of C9-C14 PFCAs, in fluoroplastics and fluoroelastomers that contain perfluoroalkoxy groups. All emissions of C9-C14 PFCAs during the manufacture and use of fluoroplastics and fluoroelastomers that contain perfluoroalkoxy groups shall be avoided and, if not possible, reduced as far as technically and practically possible. This derogation shall not apply to articles referred to in paragraph 2(c). The Commission shall review this derogation no later than 25 August 2024.
- The concentration limit referred to in paragraph 2 shall be 1 000 ppb for the sum of C9-C14 PFCAs, where these are present in PTFE micro powders produced by ionising irradiation or by thermal degradation, as well as in mixtures and articles for industrial and professional uses containing PTFE micro powders. All emissions of C9-C14 PFCAs during the manufacture and use of PTFE micro powders shall be avoided and, if not possible, reduced as far as technically and practically possible. The Commission shall review this derogation no later than 25 August 2024.
- For the purposes of this entry, C9-C14 PFCA-related substances are substances that, based on their molecular structure, are considered to have the potential to degrade or be transformed to C9-C14 PFCAs.’
Now that we went through the restriction and all the derogations, let me write what should you do about it.
Firstly, you need to identify if the concerned substances are present in your products. If you are a distributer, you should get this information from higher up the supply chain.
Secondly, check if your products fall under derogation with later deadlines than 25 February 2023.
Thirdly, if your products fall under the derogation, then you can follow the derogation.
If no derogation can be applied to your products, then you may not use them or place them on the market anymore after 25 February 2023, according to the restriction.
If you need consulting regarding this vast restriction, we can help you. Just drop us an e-mail at info@bens-consulting.eu.






 Back to posts
Back to posts

