Let's have a look today at the third condition (c), which – as opposed to the first two – pertains to the exact concentration of an ingredient.
This condition states that an exact ingredient concentration has been submitted, followed by modification of the concentration exceeding the ranges stated in Table 3.
Table 3 below contains guidelines and limits that need to be taken into account when assessing whether a new UFI is needed:
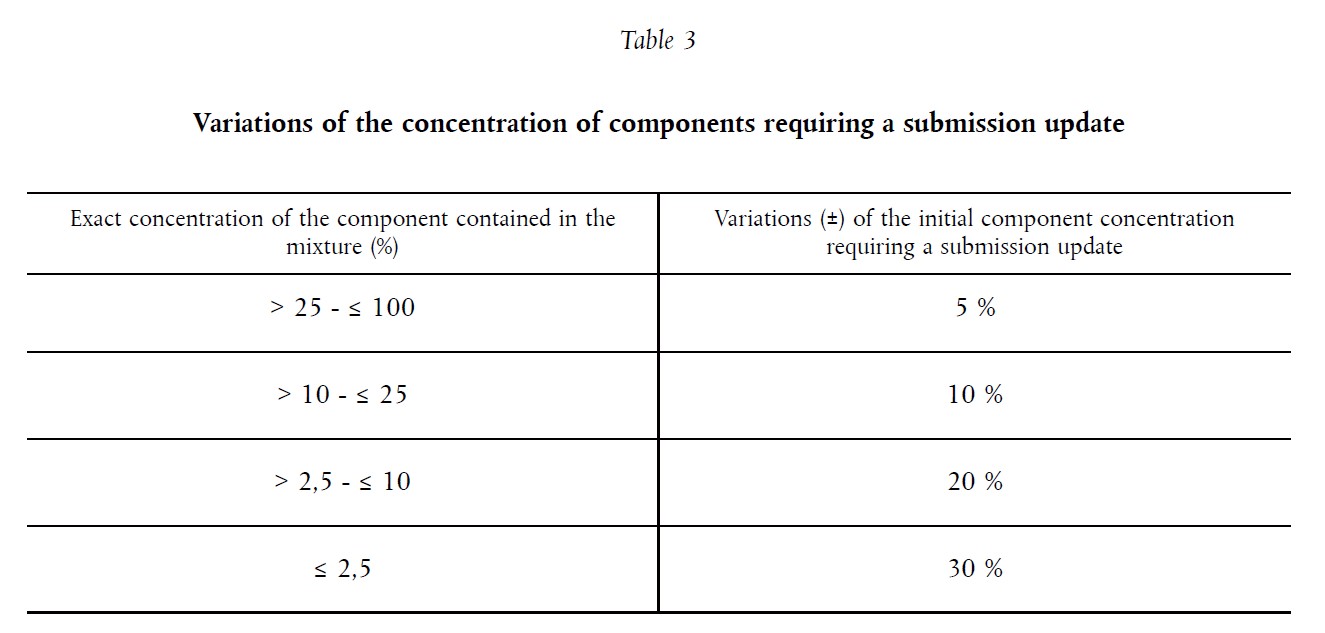
Let’s have a look at a few examples for better understanding:
- Concentration of the substance classified with H319 is 20 %. This concentration was also submitted in the first submission (the exact concentration was used instead of a range). New concentration is 25 %.
Answer: According to the table 3, the maximum modification of the initial concentration is ±10 %. The new concentration represents a 25 % change. Therefore, a NEW UFI must be generated.
- Concentration of substance is 72 %. This concentration was submitted in the first submission. Answer: According to Table 3, a maximum discrepancy of ± 5 % is allowed. In this case, a new UFI must be generated if the new concentration of the substance is below 68.4 % or above 75.6 %.
The compound contains 4 substances (A, B, C, D). As you can see in the table below (second column), their exact concentration was submitted.
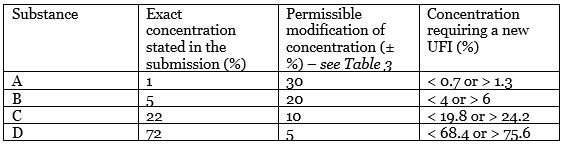
The third column in turn contains permissible discrepancies in relation to the previously stated exact concentration. It means that you need to create a new UFI if concentration changes below or above the value stated in the last column.
I hope the examples are clear enough, and helped you understand the requirements related to UFI. In any case, you can contact me and we will have a chat about your particular challenge.
Here are the links to the first and second post on this topic.






 Back to posts
Back to posts

